A 65-year-old patient with stage III NSCLC comes to your clinic to discuss concurrent chemoradiotherapy. She has a history of ischemic heart disease, and 4 years ago an angiogram demonstrated minor coronary artery disease that did not require intervention. Her angina is well-controlled on medication, and she only rarely uses her glyceryl trinitrate spray. She is active, enjoys gardening, and has a performance status of 1.
This scenario is not uncommon; at least one-quarter of patients treated for lung cancer have pre-existing cardiovascular disease (CVD). Given improved outcomes for patients with lung cancer treated with curative-intent radiotherapy (particularly with the introduction of immunotherapy in the stage III NSCLC setting) and growing evidence regarding the impact of heart dose on survival, radiation-related cardiac toxicity has become an area of increased research interest.1
The patient’s situation described above raises a number of questions about the cardiac effects of thoracic radiotherapy. First, what is the risk of cardiac toxicity with chemoradiotherapy, and is it increased for patients with CVD? Second, what dose limits should be used to protect the heart, and should these limits be applied to the whole heart or only to certain cardiac substructures? Finally, what other interventions can be applied to reduce her risk of cardiac-related side effects?
A recent paper in the Journal of Thoracic Oncology (JTO) written by a multidisciplinary team of oncologists, scientists, and cardiologists on behalf of the IASLC Advanced Radiation Technology Committee summarizes the growing evidence around the cardiac toxicity of radiotherapy in patients with lung cancer and provides some answers to the questions above.1
Radiation-induced heart disease covers a wide range of conditions that can occur following thoracic radiation. These conditions arise because of acute inflammation leading to microvascular damage, chronic inflammation, and fibrosis of cardiac substructures. The resulting pathology depends on which cardiac substructure is damaged (Fig.).
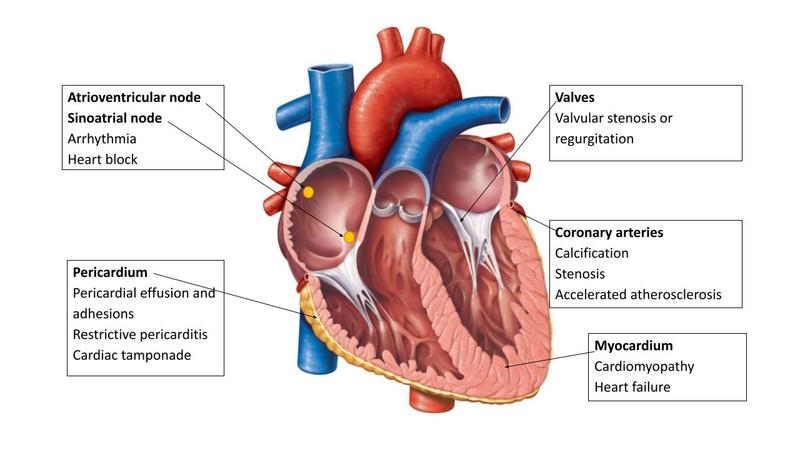
Darby et al.2
were the first to show that cardiac events increase with increasing mean heart dose in patients who receive radiotherapy for breast cancer, with no safe minimum dose. A similar result has been shown in patients receiving radiotherapy for mediastinal Hodgkin lymphoma.3
Nevertheless, the results of RTOG 0617 and other studies demonstrate that there are major differences in impact of radiation on the heart between patients who have thoracic radiotherapy for breast cancer or lymphoma and those who have radiotherapy for lung cancer.
The JTO article summarizes studies investigating the relationship between heart dose and survival and the impact of pre-existing CVD. Some studies have shown an increased rate of cardiac events following thoracic radiotherapy in patients with a past medical history of CVD, although a study by Atkins et al.4
showed that mean radiation dose to the heart was not associated with major adverse cardiac events in patients with a history of CVD. Conversely, patients with no history of CVD in this study had an increased rate of cardiac events with a mean heart dose of more than 10 Gy, indicating that cardiac dose could be more relevant for patients without a history of CVD.
Instead of investigating dose to the whole heart, it may be more relevant to study dose to cardiac substructures. Although this question is currently the focus of a number of clinical studies, to date there are no consistent cardiac substructure dose limits routinely in use in clinical practice. The most consistent data relate to the dose to the base of the heart (incorporating the atria, origin of the coronary arteries, and aortic root), which has been shown in a number of studies to be associated with reduced OS.
There are limitations with existing studies on cardiac toxicity of thoracic radiotherapy as well as a lack of consistency in the recording of pre-existing cardiac disease and cardiac endpoints and in cardiac contouring. The most commonly used atlases for heart contouring are the Feng atlas5
(which was developed based on the experience of patients undergoing breast radiotherapy) and the Kong6
atlas, which does not cover cardiac substructures in detail. Differences in cardiac contouring between published references will have a major impact on dose reporting and comparison of outcomes. It is also important to consider the systemic therapy that the patient will receive alongside radiotherapy. Cisplatin, although not directly cardiotoxic, can cause endothelial dysfunction and platelet activation, leading to ischemia and thrombosis. Checkpoint inhibitors can cause myocarditis and cardiac arrhythmias.
Advanced radiotherapy techniques can be used to reduce heart dose. Proton beam radiotherapy may reduce heart dose for some patients, but dose to cardiac substructures directly adjacent to the tumor may be increased in others. At present, although there are data showing that protons can reduce the mean heart dose, there is no evidence that this leads to a reduction in cardiac toxicity in patients treated with radiotherapy for lung cancer.7
MRI-guided radiotherapy is another treatment platform that may allow for reduction of planning target volume margins and adaptation of treated volumes, leading to reductions in dose to cardiac substructures for tumors close to the heart.
There are a number of ongoing studies evaluating the effect of some of these advanced radiotherapy technologies on cardiac toxicity and patients’ outcomes. To make progress in this field, high-quality prospective research is required, including standardized cardiac endpoints and heart contouring atlases. This would allow the development of applicable models with improved power to identify heart dose constraints and to predict factors of cardiac toxicity.
At the moment it seems that there are more questions than answers when it comes to the cardiac toxicity of thoracic radiotherapy. Going back to our patient with stage III NSCLC, what can we do for her? The JTO article lays out a few practical suggestions for improving the care of such patients. First, her cardiac health can be optimized with anti-platelet therapy; cholesterol, blood pressure, and diabetic control; and exercise and smoking cessation. Second, regular monitoring for cardiac complications and referral to a cardiologist, ideally with an interest in cardio-oncology, should be considered if the cardiac risk is deemed high. Management of cardiac complications from radiotherapy depends on the affected cardiac substructure (Table). Preclinical studies have shown that statins, in addition to lowering cholesterol, reduce the inflammation associated with radiation, and angiotensin-converting enzyme inhibitors prevent adverse cardiac remodeling. Third, active breathing control can increase lung capacity and reduce tumor motion and cardiac dose by moving the tumor away from the heart. Finally, daily on-treatment cone-beam CT imaging with a low threshold for correction of set-up errors has been shown to improve the survival of patients treated with radiotherapy for lung cancer.8
This strategy of improved image-guided radiotherapy and optimal cardiac care is achievable for most patients. In the future, we believe that more prospective studies with consistent cardiac contouring and clear outcome reporting will improve our knowledge of how best to avoid and treat cardiac toxicity in thoracic radiotherapy.
| Pathology | Symptoms and Signs | Investigation | Management |
|---|---|---|---|
| Pericardium | |||
| Acute pericarditis | Fever, Chest pain, pericardial rub | Echo, CMR | Symptomatic pain relieve with anti-inflammatory medications (e.g., NSAIDs or aspirin) Colchicine |
| Pericardial effusion | Dyspnea, cardiac tamponade, quiet heart sounds | Serial echo | Pericardiocentesis if patient acutely unwell secondary to cardiac constriction/tamponade |
| Constrictive pericarditis | Dyspnea, edema, fatigue, pericardial rub | Echo, CMR, CCT to identify calcification |
Diuretics if heart failure present Surgery in intractable cases |
| Myocardium | |||
| Cardiomyopathy and heart failure | Dyspnea, edema, fatigue, cough |
Blood NT pro-BNP Echo, CMR |
Diuretics, beta blockers, ACE inhibitors, angiotensin receptor blockers/angiotensin receptor–neprilysin inhibitors |
| Coronary Arteries | |||
| IHD | Chest Pain |
Blood Troponin levels ECG Echo OCT Angigraphy |
Cardiac risk-facotr optimization and secondary prevention with statins and aspirin Beta blockers, Ca-channel blockers Anit-anginals (e.g., nitroglycerine, ivabradine, ranolazine, nicorandil) Re-vascularization if high symptom burden or significant stenosis of left main stem/proximal left anterior descending |
| Valves | |||
| Regurgitation and stenosis | Dyspnea, edema, fatigue, cough, chest pain, cardiac murmur |
Echo CMR OCT |
Diuretics, anti-coagulation, blood pressure control Valve replacement with surgery or TAVI |
| Conduction System | |||
| 14Arrhythmia | Palpitations, dizziness, dyspnea, chest pain |
ECG (ambulatory) Echo CMR |
Anti-arrythmics Pacemaker Cardiac resynchronization |
- 1. a. b. Banfill K, Giuliani M, Aznar M, et al. Cardiac Toxicity of Thoracic Radiotherapy: Existing Evidence and Future Directions. J Thorac Oncol. 2020;16(2):216-227.
- 2. Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-998.
- 3. van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol. 2016;34(3):235-243.
- 4. Atkins KM, Rawal B, Chaunzwa TL, et al. Cardiac Radiation Dose, Cardiac Disease, and Mortality in Patients With Lung Cancer. J Am Coll Cardiol. 2019;73(23):2976-2987.
- 5. Feng M, Moran JM, Koelling T, et al. Development and Validation of a Heart Atlas to Study Cardiac Exposure to Radiation Following Treatment for Breast Cancer. Int J Radiat Oncol. 2011;79(1):10-18.
- 6. Kong F-MS, Ritter T, Quint DJ, et al. Consideration of Dose Limits for Organs at Risk of Thoracic Radiotherapy: Atlas for Lung, Proximal Bronchial Tree, Esophagus, Spinal Cord, Ribs, and Brachial Plexus. Int J Radiat Oncol. 2011;81(5):1442-1457.
- 7. Liao ZX, Lee J, Komaki R, et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol. 2016;34(15)(suppl):8500.
- 8. Johnson-Hart C, Price G, Vasquez Osorio E, Faivre-Finn C, and van Herk M. The impact of baseline shifts towards the heart after image guidance on survival in lung SABR patients. Radiother Oncol. 2020;152:183-188.




