Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors
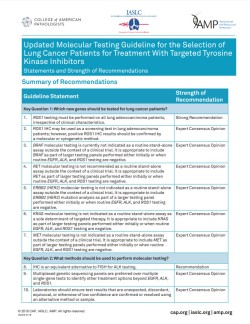
The "Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors" continues to set evidence-based standards for clinical molecular testing of non-small cell lung cancers (NSCLC) that effectively guides targeted therapy and treatment.
This guideline was endorsed by the American Society of Clinical Oncology on February 5, 2018.
Learn More About Molecular Testing
Molecular Testing Guideline Video for Physicians
Molecular Testing Guideline Video for Patients

Pathology Committee
The Pathology Committee is very involved in the creation of testing resources and educational content, from the new Immunohistochemistry Atlas to ILCN articles about timely and nuanced topics relevant to pathology. The committee boasts a long timeline of milestone achievements.