The IASLC Pathology Committee plays a fundamental role in fulfilling core aspects of the association's mission. The Committee is an active working group of like-minded individuals who produce publications, contribute to and provide guidance for IASLC educational meetings, and help establish the IASLC as an international leader in the clinical and scientific aspects of lung cancer pathology. The pathology committee consists of up to 30 IASLC members who are pathologists or who specialize in pathology research. The Committee is funded by the IASLC for meetings and projects that pertain to its mission statement.

Committee projects
Chair of Committee

Fernando Lopez-Rios
2025-2027
2025-2027 Roster
Committee Responsibilities:
Committee Contributions and Milestones
Since its founding in 1982, the IASLC Pathology Committee has made a series of major contributions to the development of the discipline and the overall treatment of lung cancer and other thoracic malignancies.
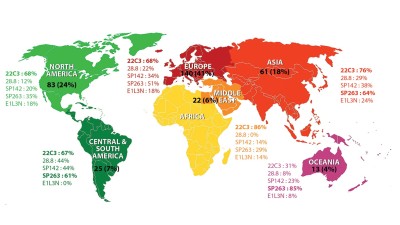
In an effort to encapsulate this work, the IASLC has compiled an interactive infographic that traces both the work of the committee and select landmark events in the field of pathology.